
Using Gases
High-purity gases for gas chromatographic trace analysis
gas aktuell No. 30, 1985
Photo: Gas mixtures are tested for the correct composition with gas chromatographs.

Using Gases
High-purity gases for gas chromatographic trace analysis
gas aktuell No. 30, 1985
Photo: Gas mixtures are tested for the correct composition with gas chromatographs.
It takes gases of the highest purity and proper high-purity gas supply systems to achieve the highest sensitivities in gas chromatography. The choice of carrier gas also depends on the operating principle of the detector. Tests with contaminated carrier gases showed how chromatograms are distorted and how to prevent errors.
A gas chromatograph comprises the following elements:
- Carrier gas with pressure regulator and transfer unit,
- Sample delivery system (gas metering valve, injection block),
- Separation system comprising separation column, separation capillary or column switching with temperature control,
- Detector,
- Signal processing (amplifier, integrator, recorder, computer).
Key factors affecting the sensitivity and the attainable detection limits include the choice of detector and the associated electronic systems as well as the purity of the carrier gas that is suitable for the detector.
Role of the carrier gas
The carrier gas must fulfill different functions in gas chromatographs: It transports the sample to the separation system. There if forms the “mobile phase” (gas phase), which also participates in the distribution processes with the “stationary phase” (separation column fill material). The choice of the separation system determines the separation of the sample – depending on the polarity, molecular weight or boiling point of the individual components of the sample. Therefore, the choice of carrier gas depends on the transport-determining gas properties, on density and viscosity. Furthermore, solubility and adsorption properties of the carrier gas must be taken into account in the stationary phase.
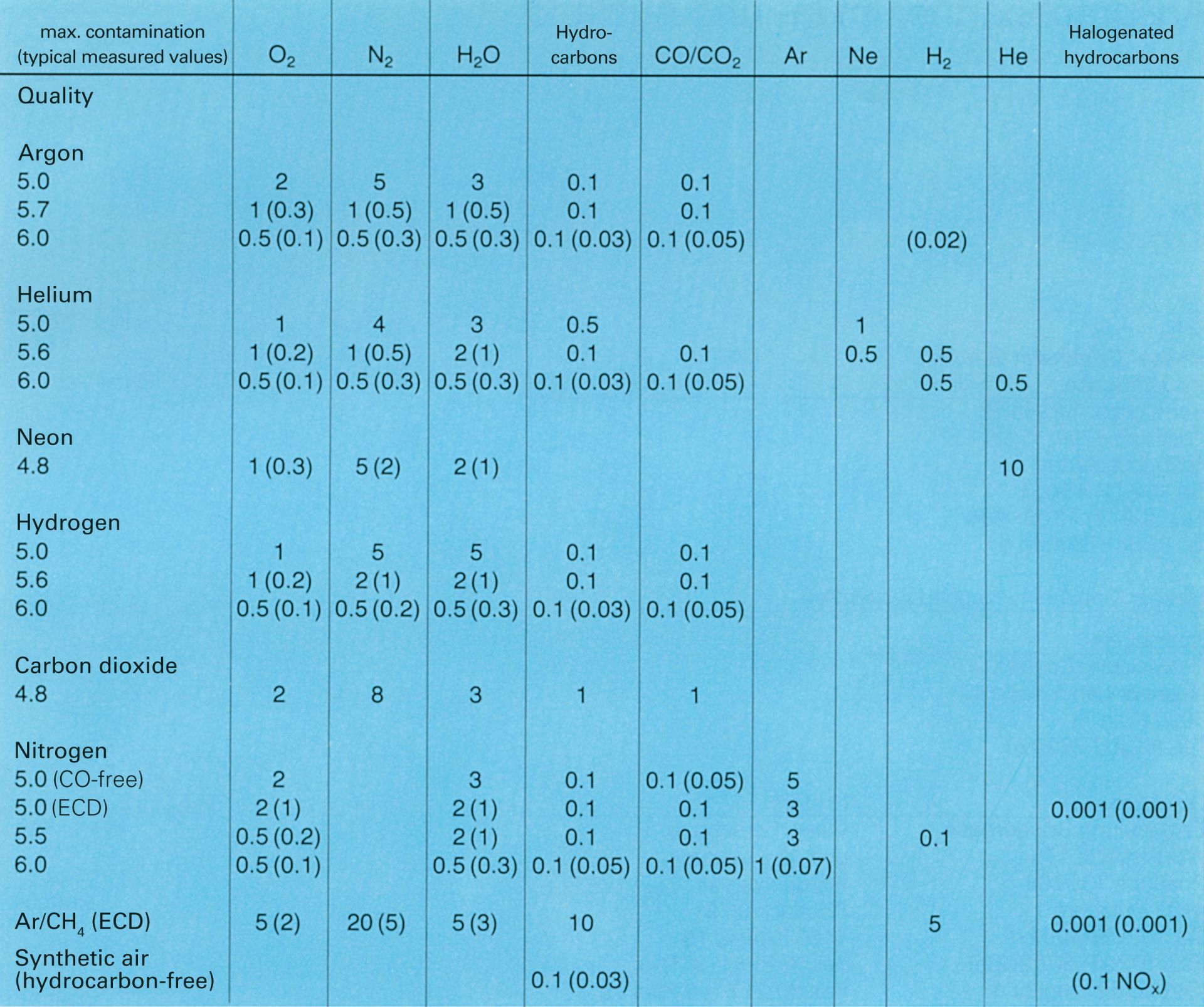
Maximum allowable contamination levels of carrier gases for chromatography in ppm; the values in parentheses are typical measured values, which are significantly below the allowable limits.
After that, the carrier gas transports the individual components to the detector, where it participates in the physical or chemical process (depending on the detector principle) to detect the components. The aim is to obtain the lowest possible background noise (high sensitivity), a linear signal (good calibration ability) and a large dynamic range. As a result, the carrier gas is selected according to the analytical task and the type and operating principle of the appropriate detector.
Contaminated carrier gases distort the measurement
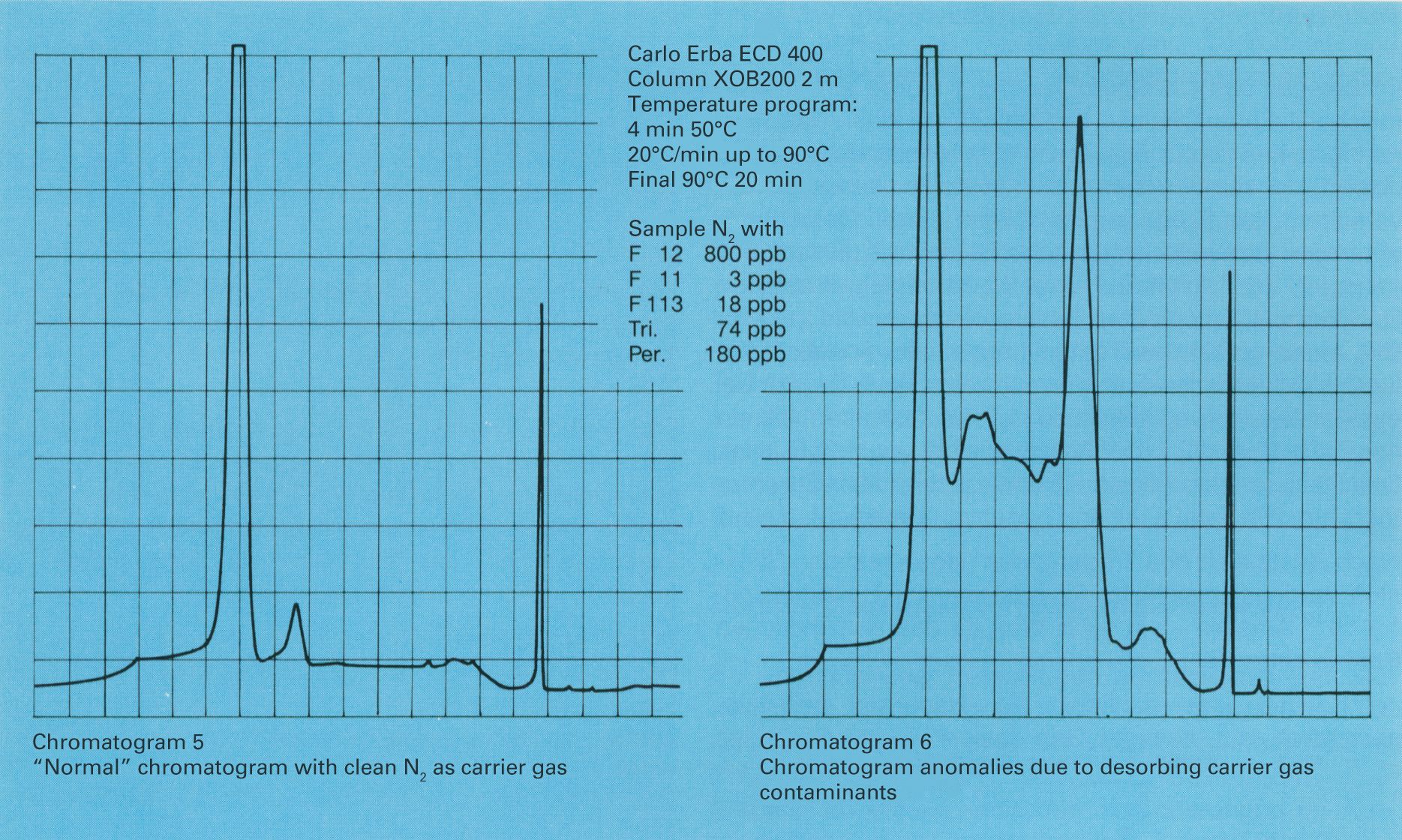
Insufficient purity of the carrier gas causes anomalies in the chromatogram, the sources of which are often hard to identify. In the different functional components of the gas chromatograph, the impurities can lead to various changes in the output signal or the chromatogram. Carrier gas impurities have another effect on the chromatogram when the separation system follows a programmed temperature profile, in order to display high boiling components of the sample together with the volatile components in one chromatogram. During the cool-down phase of the separation system between analyses, trace impurities can concentrate at the column inlet. In the next analysis, those concentrated components overlap with the chromatogram. Electron capture detectors (ECDs) can detect certain electron-absorbing substances, such as halogenated hydrocarbons, with extreme sensitivity. ECDs are typically operated with pulsed voltage, whereby the pulse frequency is changed to maintain constant ion flow in the detector. The lowest achievable frequency (with ion flow adjusted) is a good criterion for the sensitivity of the ECD. It is directly dependent on the purity of the carrier gas.
Proper high-purity gas supply systems
For supplying high-purity carrier gases to the gas chromatograph, a properly installed high-purity gas supply system is needed, in order to prevent subsequent contamination of the gases. Gas chromatography cannot provide optimal analyses unless the interaction of multiple system components functions correctly: to the gas chromatograph, with the same requirements, the high-purity carrier gas and the proper gas supply system for that gas.

